Explained: LEL vs. UEL what’s the difference?
Everyone who had an emergency response course at their company has heard this a hundred times; to create a fire or explosion, 3 components are needed simultaneously: a flammable substance (gas, vapour or dust), oxygen and ignition.
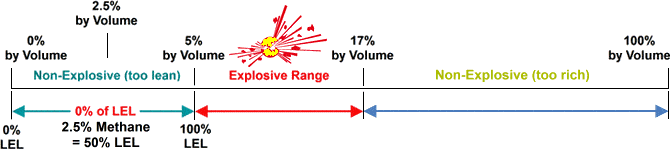
A fuel (i.e. combustible gas) and oxygen (O2) must be present in specific ratios, along with an ignition source, such as a spark or flame. The required fuel and oxygen ratio varies with each combustible gas or vapour. That’s where LEL (lower explosive level) and UEL (upper explosive level) come into the equation.
LEL vs. UEL
The minimum required amount of a particular combustible gas or vapour necessary to support its combustion in air is defined as the Lower Explosive Limit (LEL) for that specific gas. Below this level, the mixture of gas and oxygen is too “thin” to burn. The maximum amount of gas or vapour that will burn in the air is the Upper Explosive Limit (UEL). Above this level, the mixture is too “rich” to burn. The scope between LEL and UEL is known as the flammable range for that gas or vapour.
source: wermac.org
LEL and UEL per type of gas
The LEL and UEL of combustible gases differ per type. The values shown in the table below represent the conditions under which they were determined (room temperature and atmospheric pressure using a 2-inch tube with spark ignition). The flammability range of most materials expands as temperature, pressure and container diameter increase. All concentrations in percent by volume.
| Gas | LEL | UEL |
| Acetone | 2.6 | 13 |
| Acetylene | 2.5 | 100 |
| Acrylonitrile | 3 | 17 |
| Allene | 1.5 | 11.5 |
| Ammonia | 15 | 28 |
| Benzene | 1.3 | 7.9 |
| 1.3 Butadiene | 2 | 12 |
| Butane | 1.8 | 8.4 |
| n Butanol | 1.7 | 12 |
| I Butene | 1.6 | 10 |
| Cis 2 Butene | 1.7 | 9.7 |
| Trans 2 Butene | 1.7 | 9.7 |
| Butyl Acetate | 1.4 | 8 |
| Carbon Monoxide | 12.5 | 74 |
| Carbonyl Sulfide | 12 | 29 |
| Chlorotrifluoro ethylene | 8.4 | 38.7 |
| Cumene | 0.9 | 6.5 |
| Cyanogen | 6.6 | 32 |
| Cyclohexane | 1.3 | 7.8 |
| Cyclopropane | 2.4 | 10.4 |
| Deuterium | 4.9 | 75 |
| Diborane | 0.8 | 88 |
| Dichlorosilane | 4.1 | 98.8 |
| Diethylbenzene | 0.8 | |
| 1.1 Difluoro 1 Chloroethane | 9 | 14.8 |
| 1.1 Difluoroethane | 5.1 | 17.1 |
| 1.1 Difluoro ethylene | 5.5 | 21.3 |
| Dimethylamine | 2.8 | 14.4 |
| Dimethyl Ether | 3.4 | 27 |
| 2.2 Dimethyl propane | 1.4 | 7.5 |
| Ethane | 3 | 12.4 |
| Ethanol | 3.3 | 19 |
| Ethyl Acetate | 2.2 | 11 |
| Ethyl Benzene | 1 | 6.7 |
| Ethyl Chloride | 3.8 | 15.4 |
| Ethylene | 2.7 | 36 |
| Ethylene Oxide | 3.6 | 100 |
| Gasoline | 1.2 | 7.1 |
| Heptane | 1.1 | 6.7 |
| Hexane | 1.2 | 7.4 |
Related
More of the same